Prototissues
Prototissues
Living tissues and organs comprise spatially interlinked consortia of specialized cells that communicate and display collective behaviours within an integrated three-dimensional environment. Mimicking these complex living ensembles through the design and construction of artificial tissue-like systems (prototissues) based on the controlled assembly and functional integration of synthetic cell-like entities (protocells) is a major challenge that has important technological implications in bottom-up synthetic biology, bioinspired tissue engineering and microscale engineering of soft machines and devices.
Programmed assembly of synthetic protocells into thermoresponsive prototissues:
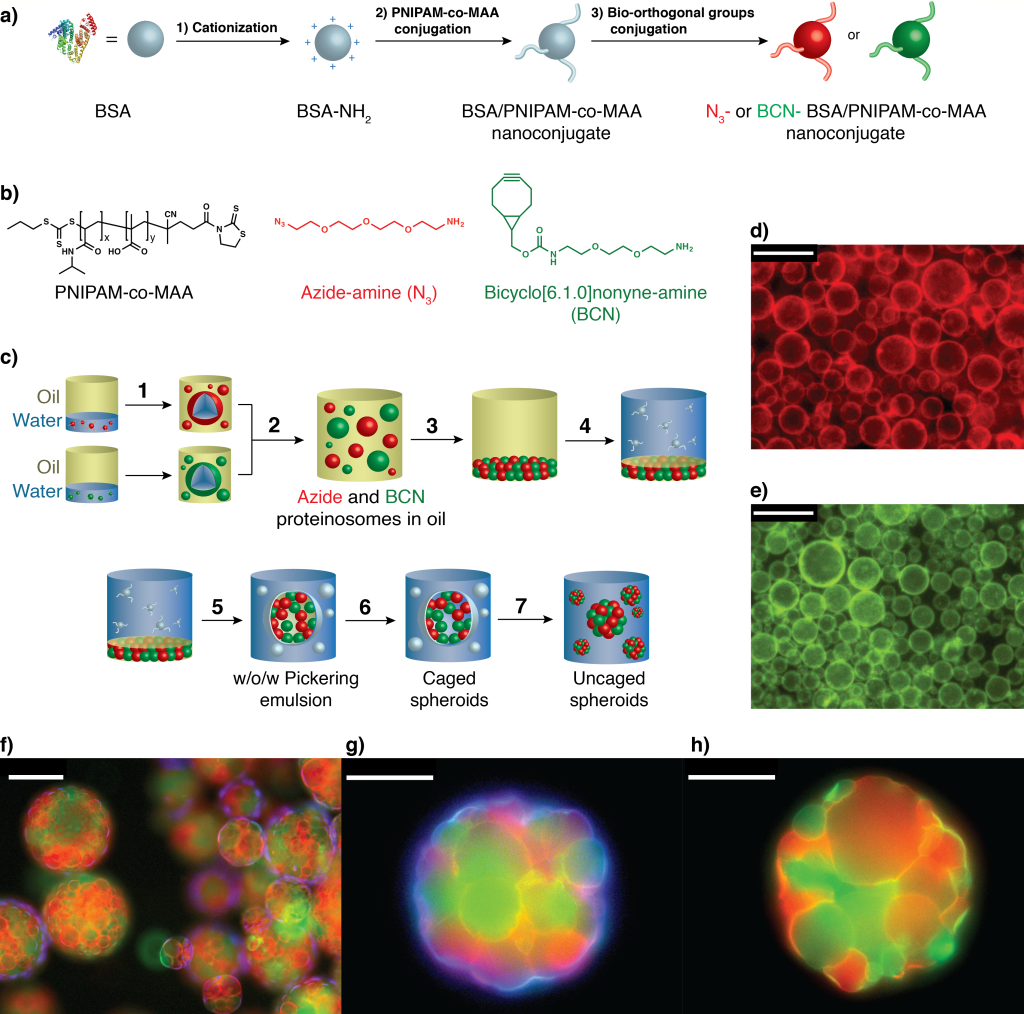
The prototissues comprise a binary community of bio-orthogonally linked proteinosome-based protocells capable of thermoresponsive collective behaviours such as enzymatically modulated reversible contraction, and mechanochemical transduction. These behaviours are based on coordinated interactions between multiple chemically coupled proteinosomes assembled at equilibrium and are therefore considered to be collective in the general sense rather than as a consequence of non-equilibrium (active) behaviour.
Key references:
- Pierangelo Gobbo, Avinash J Patil, Mei Li, Stephen Mann, Nature Materials 2018, 17, 1145–1153.
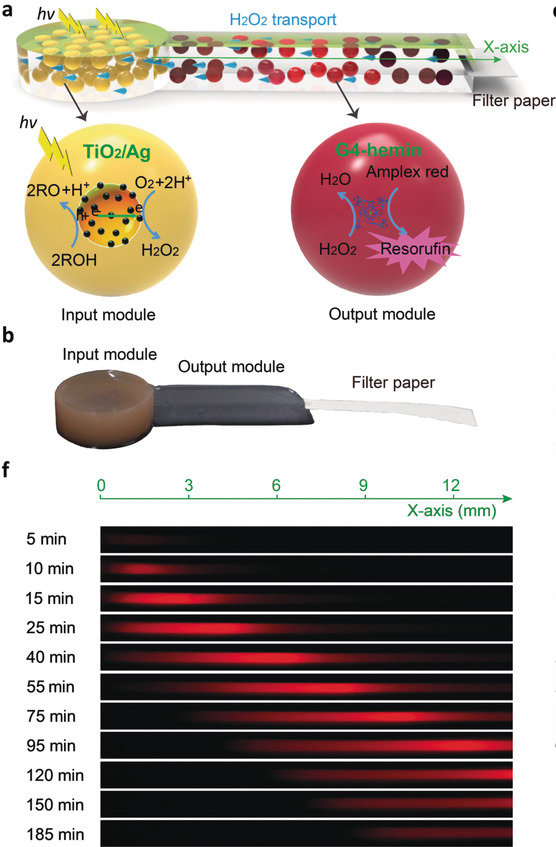
Immobilization of compartmentalized microscale objects in 3D hydrogels provides a step towards the modular assembly of soft functional materials with tunable architectures and distributed functionalities. Herein, we report the use of a combination of micro‐compartmentalization, immobilization, and modularization to fabricate and assemble hydrogel‐based microreactor assemblies comprising millions of functionalized polysaccharide–polynucleotide coacervate droplets. The heterogeneous hydrogels can be structurally fused by interfacial crosslinking and coupled as input and output modules to implement a UV‐induced photocatalytic/peroxidation nanoparticle/DNAzyme reaction cascade that generates a spatiotemporal fluorescence read‐out depending on the droplet number density, intensity of photoenergization, and chemical flux. Our approach offers a route to heterogeneous hydrogels with endogenous reactivity and reconfigurable architecture, and provides a step towards the development of soft modular materials with programmable functionality.
- Liu J, Tian L, Qiao Y, Zhou S, Patil A J, Wang K, Li M and Mann S, Angew. Chem. Int. Ed., 2020, 59, 6853-6859
Signal processing and generation of bioactive nitric oxide in a model prototissue:
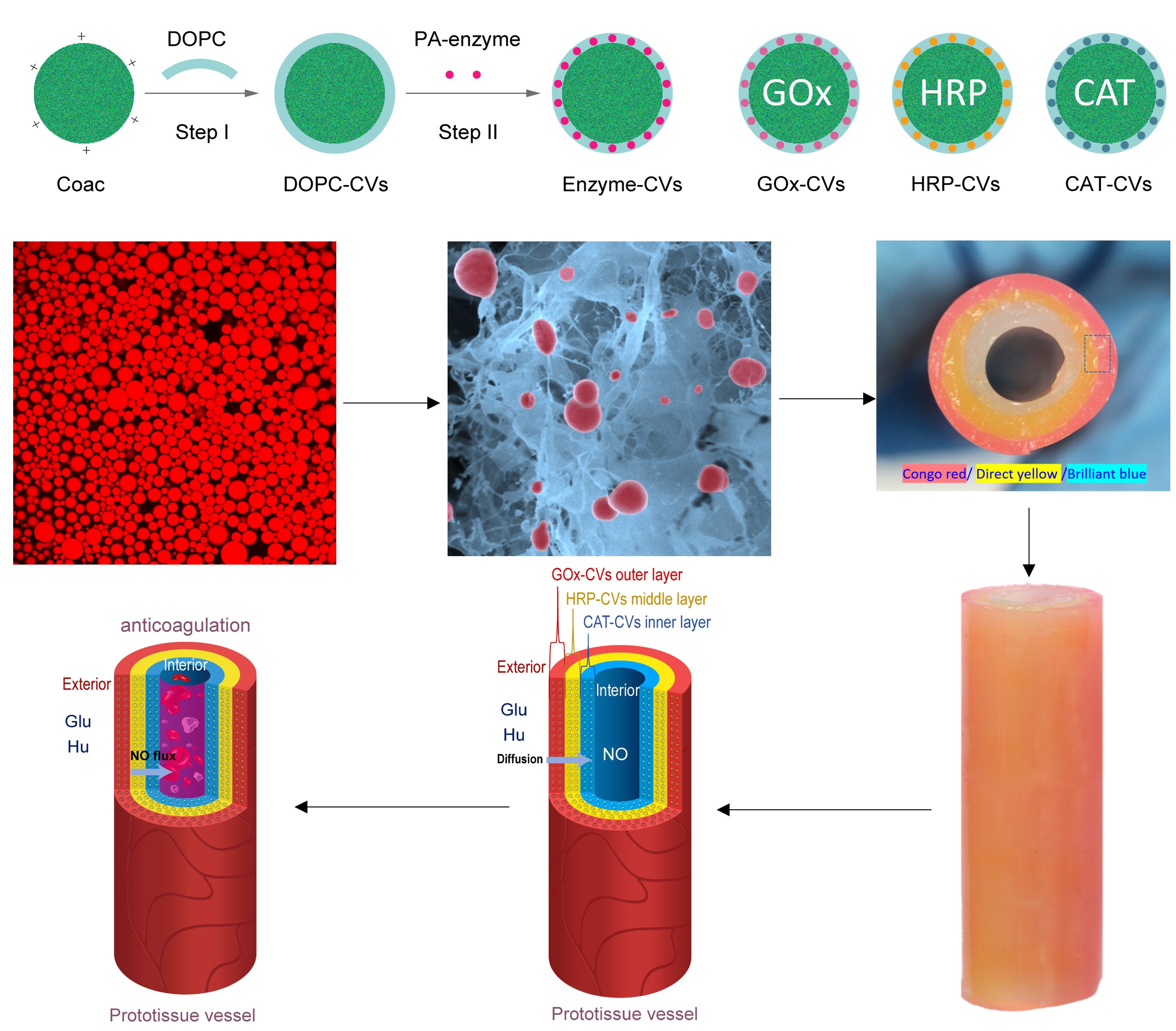
The design and construction of synthetic prototissues from integrated assemblies of artificial protocells is an important challenge for synthetic biology and bioengineering. Here we spatially segregate chemically communicating populations of enzyme-decorated phospholipid-enveloped polymer/DNA coacervate protocells in hydrogel modules to construct a tubular prototissue-like vessel capable of modulating the output of bioactive nitric oxide (NO). By decorating the protocells with glucose oxidase, horseradish peroxidase or catalase and arranging different modules concentrically, a glucose/hydroxyurea dual input leads to logic-gate signal processing under reaction-diffusion conditions, which results in a distinct NO output in the internal lumen of the model prototissue. The NO output is exploited to inhibit platelet activation and blood clot formation in samples of plasma and whole blood located in the internal channel of the device, thereby demonstrating proof-of-concept use of the prototissue-like vessel for anticoagulation applications. Our results highlight opportunities for the development of spatially organized synthetic prototissue modules from assemblages of artificial protocells and provide a step towards the organization of biochemical processes in integrated micro-compartmentalized media, micro-reactor technology and soft functional materials.
Key references:
- Songyang Liu, Yanwen Zhang, Xiaoxiao He, Mei Li, Jin Huang, Xiaohai Yang, Kemin Wang, Stephen Mann, Jianbo Liu, Nature Communications 2022, 13, 5254