Collective Behaviour of Protocells
Collective Behaviour of Protocells
Recent progress in the chemical construction of colloidal objects comprising integrated biomimetic functions is paving the way towards rudimentary forms of artificial cell-like entities (protocells). Materials based on consortia of micro-compartmentalized colloidal objects could have potential use in synergistic sensing systems and biomimetic systems engineering. We recently addressed the challenge of how to design micro-compartmentalized colloidal objects as a model of a synthetic protocell community exhibiting a rudimentary form of materials based colloidal objects, and provided a novel microscale engineering approach to inducing higher-order behaviour in mixed populations of synthetic protocells.
Phagocytosis-inspired behaviour in synthetic protocell communities of compartmentalized colloidal objects:
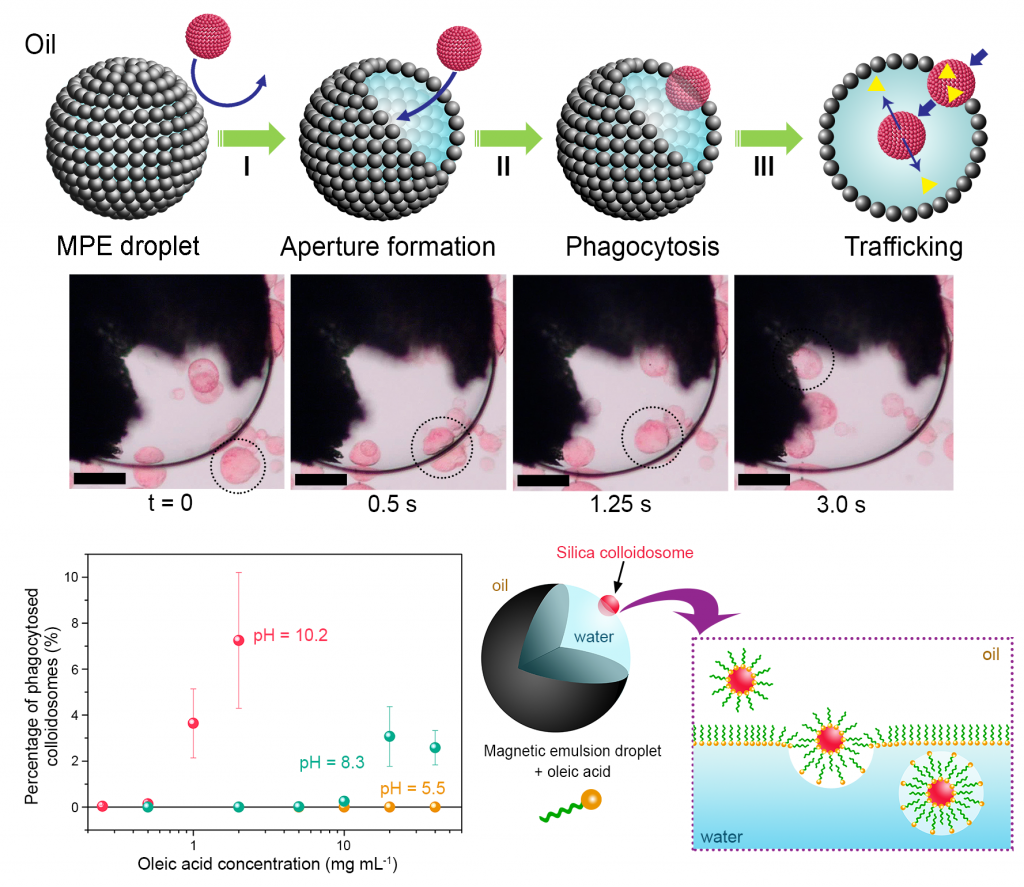
The spontaneous assembly of micro-compartmentalized colloidal objects capable of controlled interactions offers a step towards rudimentary forms of collective behaviour in communities of artificial cell-like entities (synthetic protocells). We developed a primitive form of artificial phagocytosis in a binary community of synthetic protocells in which multiple silica colloidosomes are selectively ingested by self-propelled magnetic Pickering emulsion (MPE) droplets comprising particle-free fatty acid-stabilized apertures. Engulfment of the colloidosomes enables selective delivery and release of water-soluble payloads, and can be coupled to enzyme activity within the MPE droplets.
Key references:
- Laura Rodríguez-Arco, Mei Li and Stephen Mann, Nature Materials 2017, 16, 857–863. DOI: 10.1038/NMAT4916
Predatory behaviour in synthetic protocell Communities:
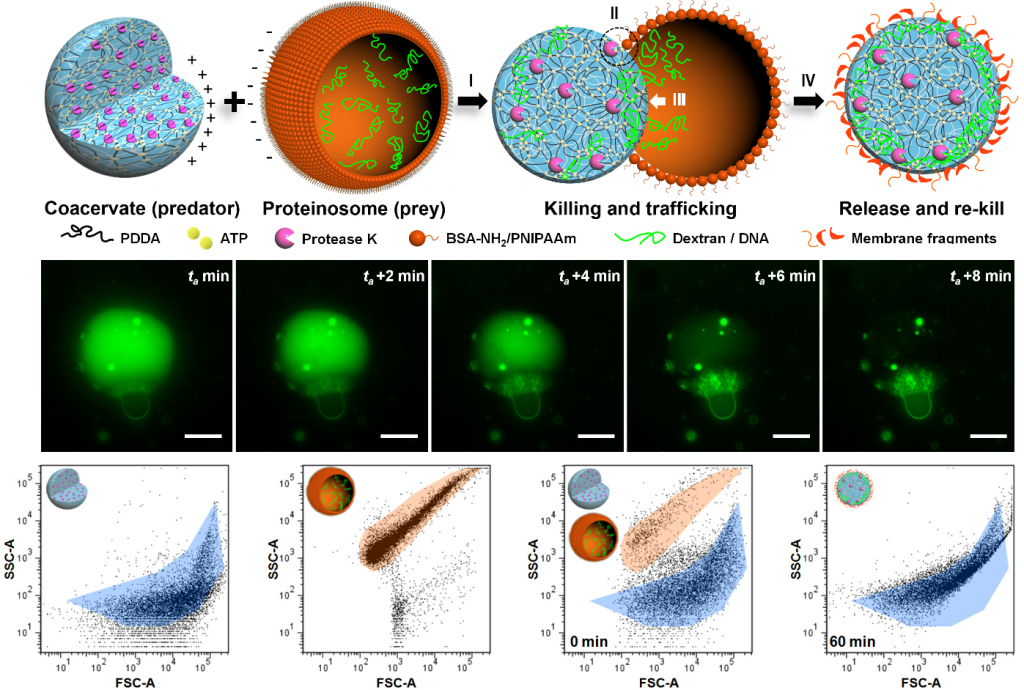
We demonstrate an artificial form of predatory behaviour in a community of protease-containing coacervate microdroplets and protein–polymer microcapsules (proteinosomes) that interact via electrostatic binding. The coacervate microdroplets act as killer protocells for the obliteration of the target proteinosome population by protease-induced lysis of the protein–polymer membrane. As a consequence, the proteinosome payload (dextran, singlestranded DNA, platinum nanoparticles) is trafficked into the attached coacervate microdroplets, which are then released as functionally modified killer protocells capable of rekilling. Our results highlight opportunities for the development of interacting artificial protocell communities, and provide a strategy for inducing collective behaviour in soft matter microcompartmentalized systems and synthetic protocell consortia.
Response‐Retaliation Behavior in Synthetic Protocell Communities:
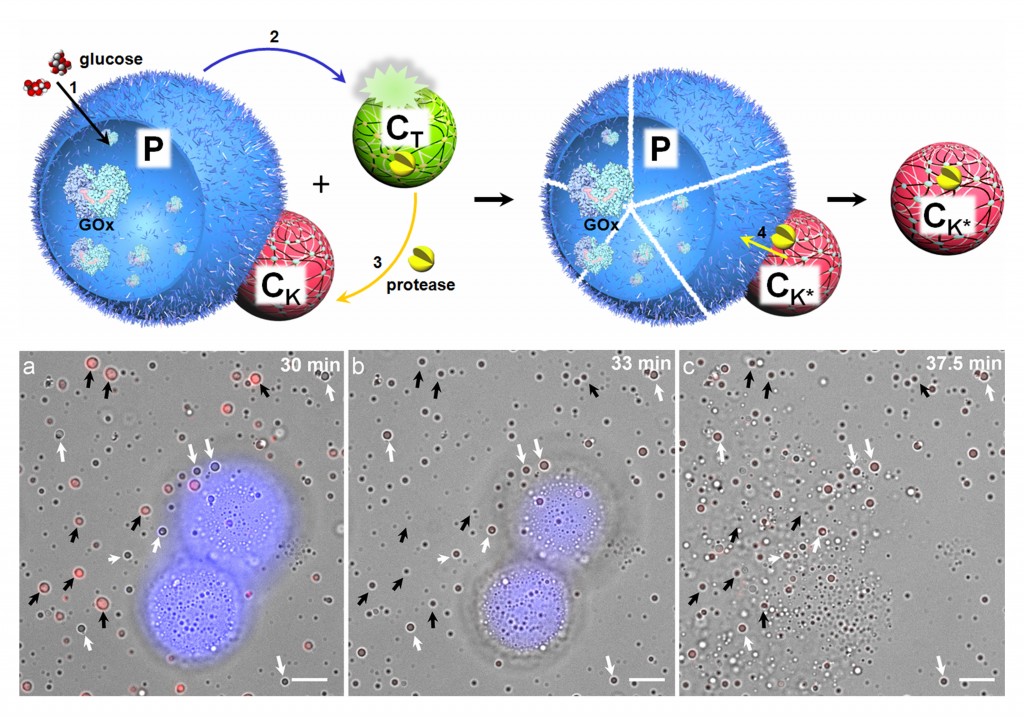
Two different artificial predation strategies are spatially and temporally coupled to generate a simple tit‐for‐tat mechanism in a ternary protocell network capable of antagonistic enzyme‐mediated interactions. The consortium initially consists of protease‐sensitive glucose‐oxidase‐containing proteinosomes (1), non‐interacting pH‐sensitive polypeptide/mononucleotide coacervate droplets containing proteinase K (2), and proteinosome‐adhered pH‐resistant polymer/polysaccharide coacervate droplets (3). On receiving a glucose signal, secretion of protons from 1 triggers the disassembly of 2 and the released protease is transferred to 3 to initiate a delayed contact‐dependent killing of the proteinosomes and cessation of glucose oxidase activity. Our results provide a step towards complex mesoscale dynamics based on programmable response‐retaliation behavior in artificial protocell consortia.
Key references:
- Yan Qiao, Mei Li, Richard Booth, Stephen Mann, Nature Chemistry, 2017, 9, 110–119. doi:10.1038/nchem.2617
- Qiao Y, Li M, Qiu D and Mann S, Angew. Chem. Int. Ed., 2019, 58, 17758-1776
Key references:
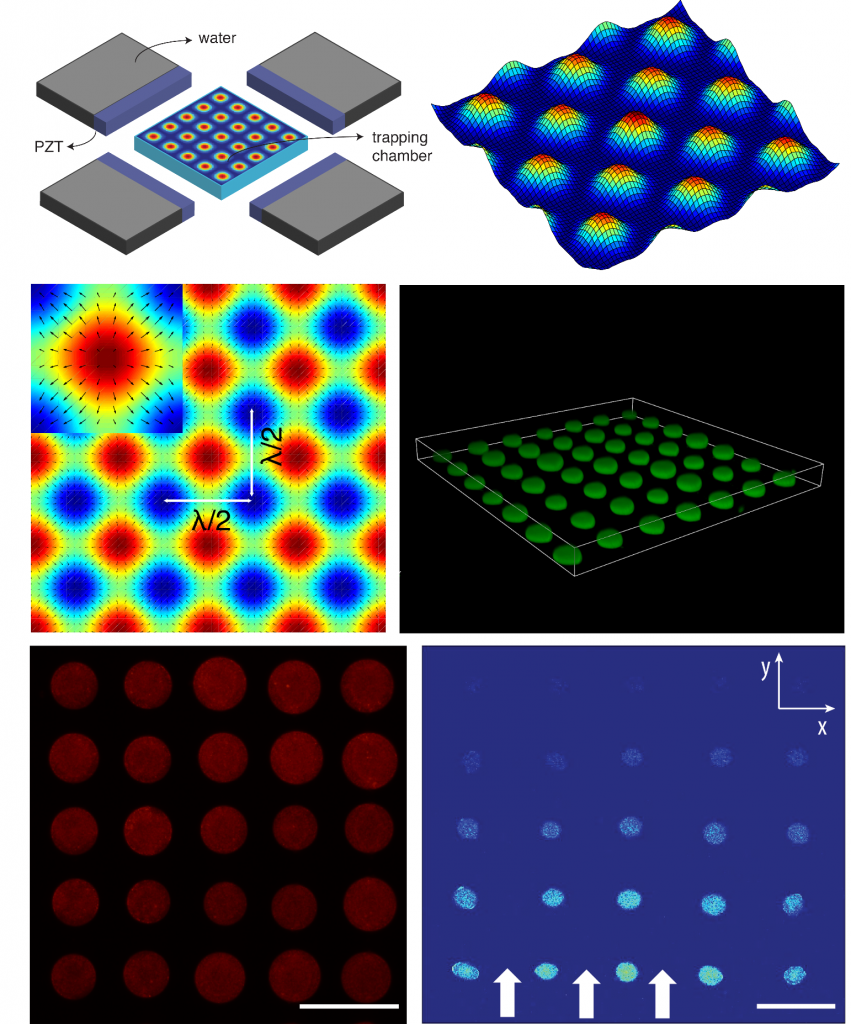
- Liangfei Tian, Nicolas C. Martin, Philip G. Bassindale, Avinash J. Patil, Mei Li, Adrian Barnes, Bruce W. Drinkwater, Stephen Mann, Nature Communications, 2016, 7:13068 doi:10.1038/ncomms13068
- Liangfei Tian, Mei Li, Juntai Liu, Avinash J. Patil, Bruce W. Drinkwater, Stephen Mann, ACS Cent. Sci., 2018, 4, 1551–1558, DOI: 10.1021/acscentsci.8b00555
- Liangfei Tian, Mei Li, Avinash J. Patil, Bruce W. Drinkwater, Stephen Mann, Nature Commun., 2019, 10, 3321. DOI: 10.1038/s41467-019-11316-4
- Xuejing Wang, Liangfei Tian, Hang Du, Mei Li, Wei Mu, Bruce W. Drinkwater, Xiaojun Han, Stephen Mann, Chem. Sci., 2019,10, 9446-9453, DOI: 10.1039/c9sc04522h
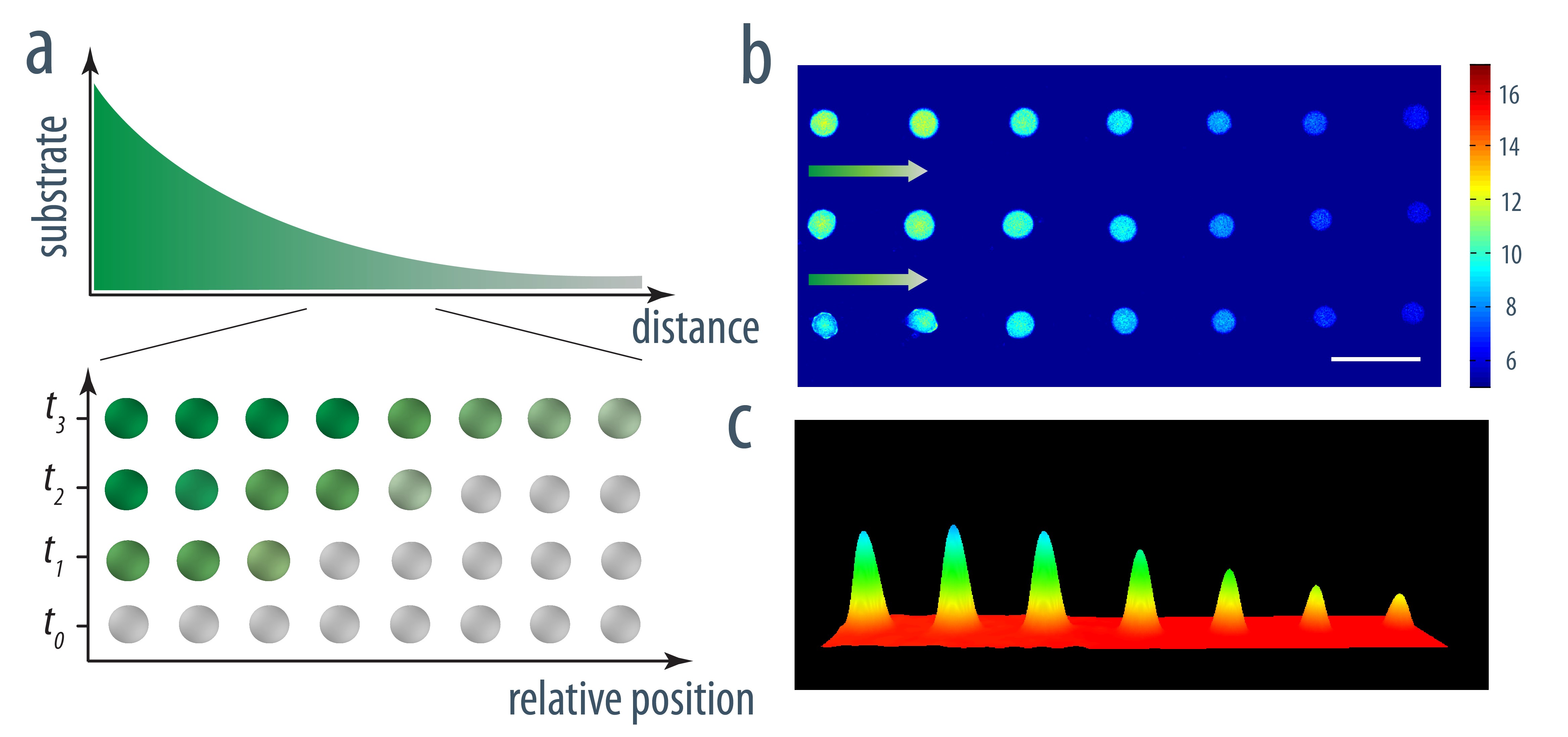
Spontaneous assembly of chemically encoded two-dimensional coacervate droplet arrays by acoustic wave patterning:
The spontaneous assembly of chemically encoded, molecularly crowded, water-rich micro-droplets into periodic defect-free two-dimensional arrays is achieved in aqueous media by a combination of an acoustic standing wave pressure field and in situ complex coacervation. Acoustically mediated coalescence of primary droplets generates single-droplet per node micro-arrays that exhibit variable surface-attachment properties, spontaneously uptake dyes, enzymes and particles, and display spatial and time-dependent fluorescence outputs when exposed to a reactant diffusion gradient. The technology of acoustic radiation forces offer a novel route to the design and construction of ‘water-in-water’ micro-droplet arrays with controllable spatial organization, programmable signalling pathways and higher order collective behaviour.
Acoustically trapped periodic arrays of horseradish peroxidase (HRP)-loaded PDDA/ATP coacervate microdroplet-based protocells exhibit a spatiotemporal biochemical response when exposed to a codiffusing mixture of substrate molecules (o-phenylenediamine (o-PD) and H2O2 under nonequilibrium conditions. Unidirectional propagation of the chemical concentration gradients gives rise to time- and position-dependent fluorescence signal outputs from individual coacervate microdroplets, indicating that the organized protocell assembly can dynamically sense encoded information in the advancing reaction-diffusion front.
Artificial morphogen-mediated differentiation in synthetic protocells:
The design and assembly of artificial protocell consortia displaying dynamical behaviours and systems-based properties are emerging challenges in bottom-up synthetic biology. Cellular processes such as morphogenesis and differentiation rely in part on reaction-diffusion gradients, and the ability to mimic rudimentary aspects of these non-equilibrium processes in communities of artificial cells could provide a step to life-like systems capable of complex spatiotemporal transformations. Here we expose acoustically formed arrays of initially identical coacervate micro-droplets to uni-directional or counter-directional reaction-diffusion gradients of artificial morphogens to induce morphological differentiation and spatial patterning in single populations of model protocells. Dynamic reconfiguration of the droplets in the morphogen gradients produces a diversity of membrane-bounded vesicles that are spontaneously segregated into multimodal populations with differentiated enzyme activities.
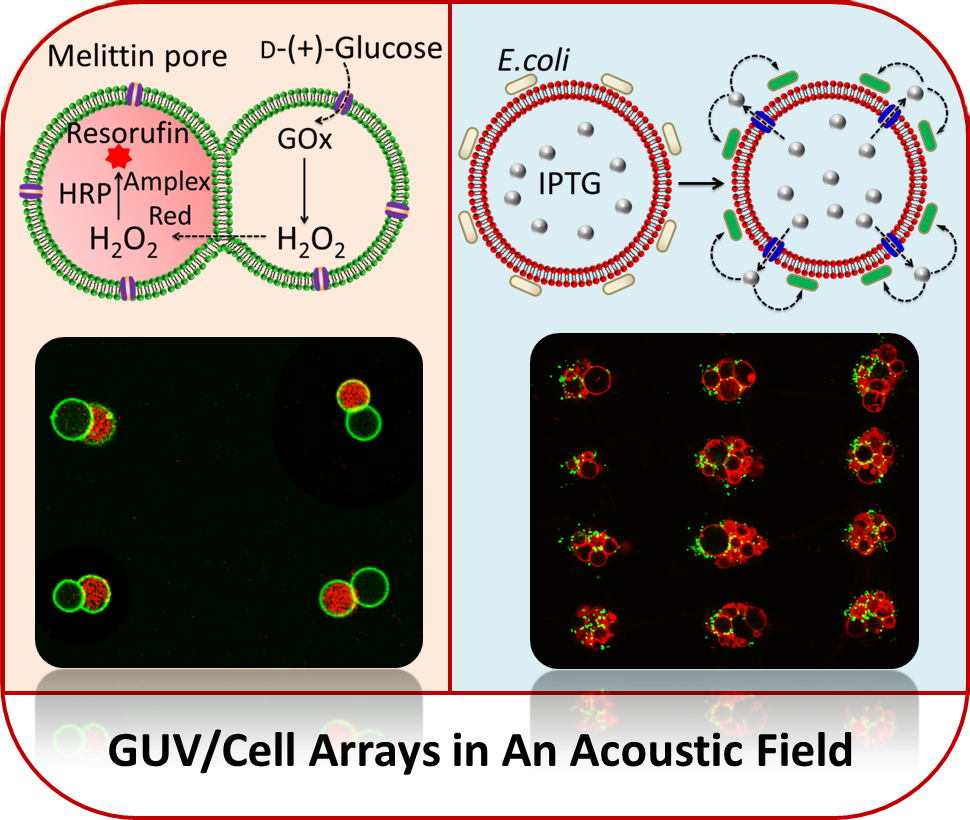
Chemical communication in spatially organized protocell colonies and protocell/living cell micro-arrays:
Micro-arrays of discrete or hemifused giant unilamellar lipid vesicles (GUVs) with controllable spatial geometries, lattice dimensions, trapped occupancies and compositions are prepared by acoustic standing wave patterning, and employed as platforms to implement chemical signaling in GUV colonies and protocell/living cell consortia. The methodology offers an alternative approach to GUV micro-array fabrication and provides new opportunities in protocell research and bottom-up synthetic biology.
Chemical signalling and functional activation in colloidosome-based protocells:
The emergence of new functionalities in inorganic colloidosomes via chemical communication between different protocell populations provides a first step toward the realization of interacting communities of synthetic functional microcompartments. An aqueous-based microcompartmentalized model involving the integration of partially hydrophobic Fe(III)-rich montmorillonite (FeM) clay particles as structural and catalytic building blocks for colloidosome membrane assembly, selfdirected membrane remodeling, and signal-induced protocell communication is described. The clay colloidosomes exhibit size- and charge-selective permeability, and show catalytic function involving peroxidase-like membrane activity, that is used for the colloidosome-mediated synthesis and assembly of a temperature-responsive poly(N-isopropylacrylamide)(PNIPAM)/clay-integrated hybrid membrane. In situ PNIPAM elaboration of the membrane is coupled to a glucose oxidase (GOx)-mediated signalling pathway to establish a primitive model of chemical communication and functional activation within a synthetic “protocell community” comprising a mixed population of GOx-containing silica colloidosomes and FeM-clay colloidosomes. Triggering the enzyme reaction in the silica colloidosomes gives a hydrogen peroxide signal that induces polymer wall formation in a coexistent population of the FeM-clay colloidosomes, which in turn generates self-regulated membrane-gated activity within the clay microcompartments.
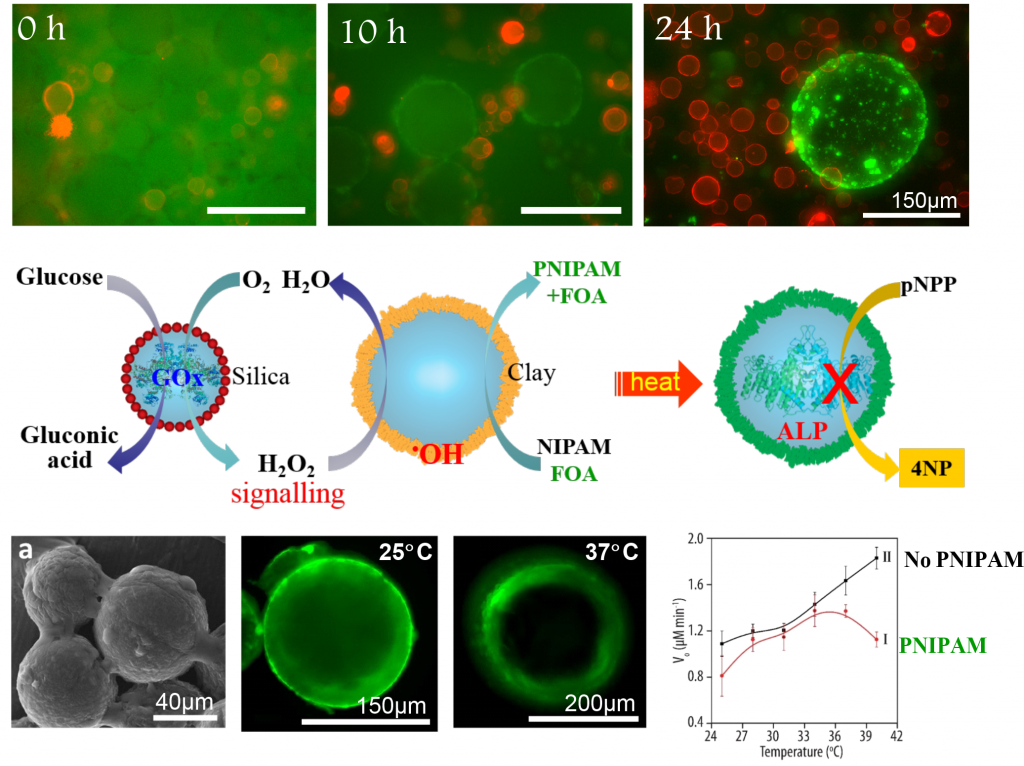
Key references:
- Shiyong Sun, Mei Li, Faqin Dong, Shengjie Wang, Liangfei Tian, Stephen Mann, Small 2016, 12, 1920–1927, DOI: 10.1002/smll.201600243
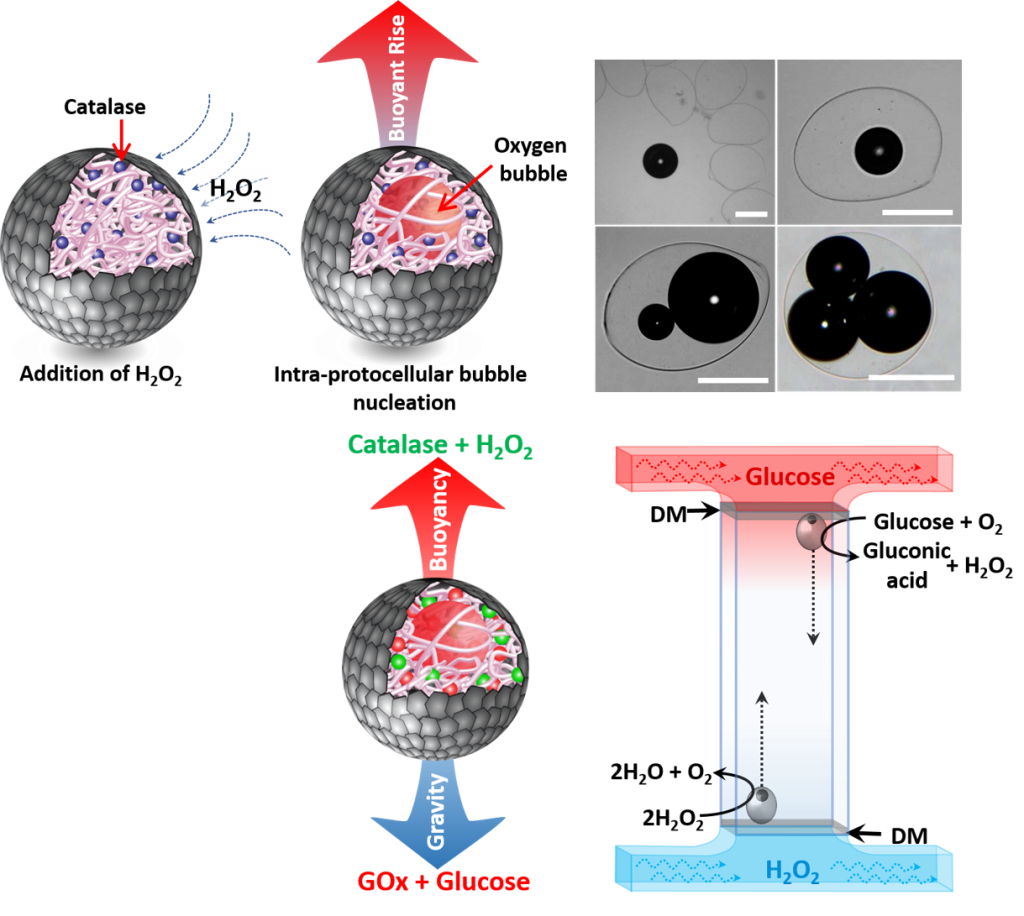
Enzyme-powered motility in buoyant organoclay/DNA protocells:
We have reported the design and construction of catalase-containing organoclay/DNA semipermeable microcapsules, which in the presence of hydrogen peroxide exhibit enzyme-powered oxygen gas bubble-dependent buoyancy. Co-encapsulation of catalase and glucose oxidase is exploited to establish a spatiotemporal response to antagonistic bubble generation and depletion to produce protocells capable of sustained oscillatory vertical movement. We demonstrate that the motility of the microcapsules can be used for the flotation of macroscopic objects, self-sorting of mixed protocell communities and the delivery of a biocatalyst from an inert to chemically active environment. This research has important implications for microcapsule-based remote sensing, environmentally induced signalling between artificial cell-like entities and programming spatial migration in synthetic protocell consortia.
Key references:
- B. V. V. S. Pavan Kumar, Avinash J. Patil, Stephen Mann, Nature Chemistry, 2018, 10, 1154–1163.
We have demonstrated the spontaneous capture of glucose oxidase-containing proteinosomes in pH-sensitive fatty acid micelle coacervate droplets as a facile route to multi-compartmentalised host–guest protocells capable of antagonistic chemical and structural coupling. The nested system functions co-operatively at low-substrate turnover, while high levels of glucose give rise to pH-induced disassembly of the droplets, release of the incarcerated proteinosomes and self-reconfiguration into spatially organised enzymatically active vesicle-in-proteinosome protocells. Co-encapsulation of antagonistic enzymes within the proteinosomes produces a sequence of self-induced capture and host–guest reconfiguration. Fabrication of compartmentalised chemical systems with nested architectures and biomimetic properties has important implications for controlling the positional assembly of functional components, spatiotemporal regulation of enzyme cascades and modelling of protoorganelle behaviour in synthetic protocells.
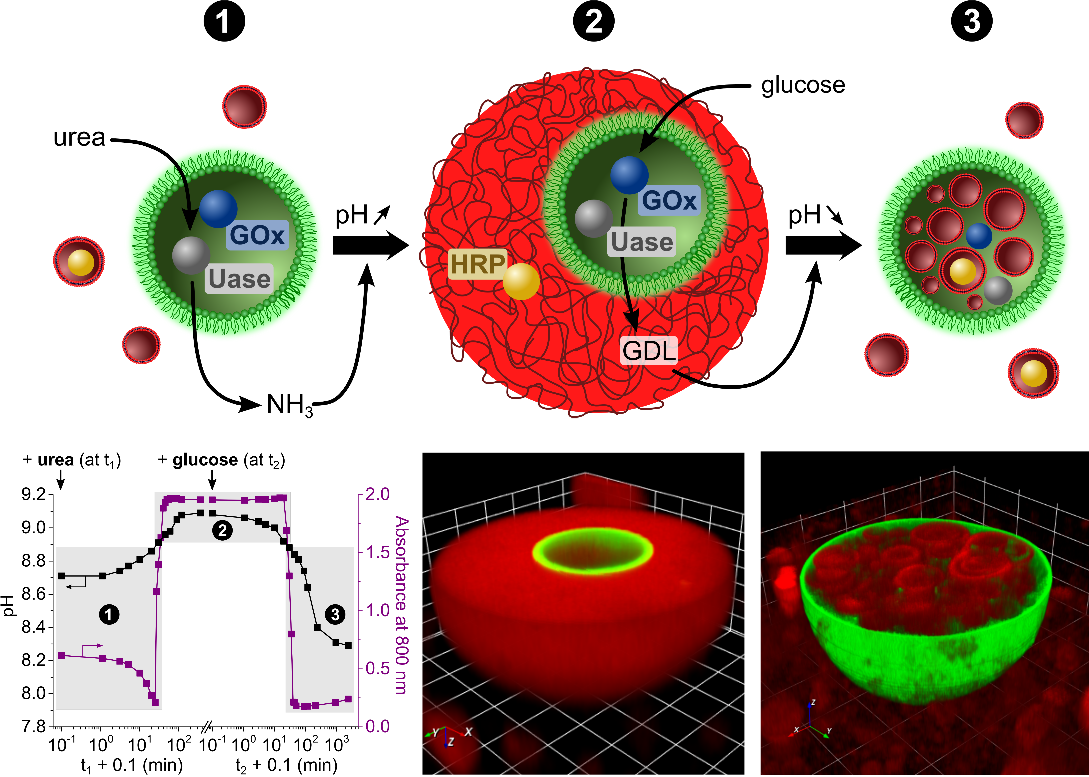
Key references:
- Martin N, Douliez J-P, Qiao Y, Booth R, Li M and Stephen Mann, Nature Communications, 2018, 9, 3652
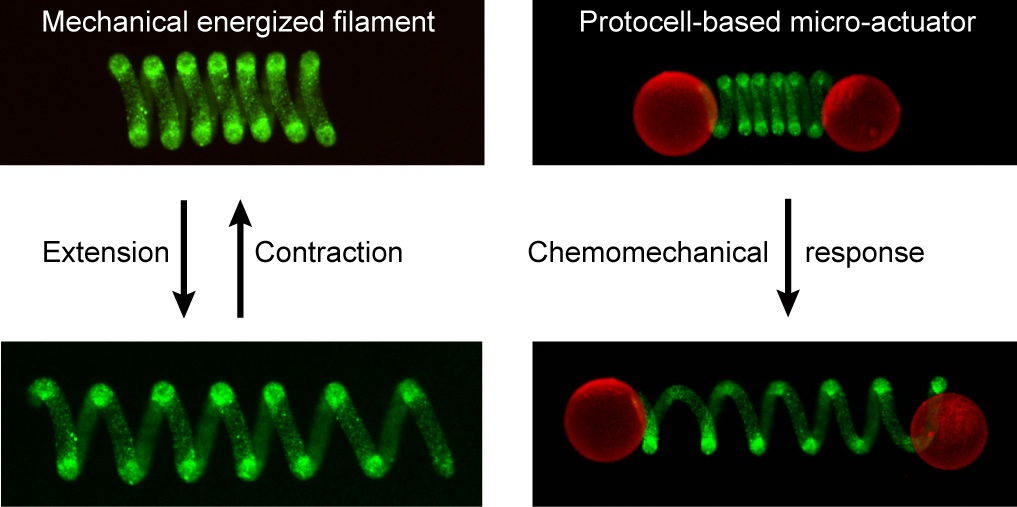
Chemical-mediated translocation in protocells-based microactuators:
Artificial cell-like communities participate in diverse modes of chemical interaction but exhibit minimal interfacing with their local environment. Here we develop an interactive microsystem based on the immobilization of a population of enzyme-active semipermeable proteinosomes within a helical hydrogel filament to implement signal-induced movement. We attach large single-polynucleotide/peptide microcapsules at one or both ends of the helical protocell filament to produce free-standing soft microactuators that sense and process chemical signals to perform mechanical work. Different modes of translocation are achieved by synergistic or antagonistic enzyme reactions located within the helical connector or inside the attached microcapsule loads. Mounting the microactuators on a ratchet-like surface produces a directional push–pull movement. Our methodology opens up a route to protocell-based chemical systems capable of utilizing mechanical work and provides a step towards the engineering of soft microscale objects with increased levels of operational autonomy.
Key references:
- Ning Gao, Mei Li, Liangfei Tian, Avinash J. Patil, B. V. V. S. Pavan Kumar, Stephen Mann, Nature Chemistry, 2021, 13, 868-879.