Hybrid Protocells
Hybrid Protocells
Although coacervate micro-droplets can be stabilized by excess surface charge, under many conditions they exhibit a propensity to coalesce into larger droplets or undergo macroscopic phase separation. As a consequence, their use as a protocell model can be compromised by their liquid-like behaviour and low surface tension. To circumvent this problem, and to generate a new type of hybrid protocell, we have recently developed several methods to produce membrane-bounded coacervate micro-droplets via spontaneous assembly or partitioning of auxiliary components on the surface of the liquid micro-compartments.
Key references:
- T-Y D.Tang, C.R.C. Hak, A.J. Thompson, M.K. Kuimova, D.S. Williams, A.W. Perriman and S. Mann, Nature Chemistry, 2014, 6, 527-533.
- M. Li, X. Huang, T.-Y. D. Tang and S. Mann, Curr. Opin. Chem. Biol., 2014, 22, 1-11.
Fatty Acid Membrane Assembly on Coacervate Micro-droplets as a Step towards a Hybrid Protocell Model:
We are exploring the use of simple physical and chemical processes to generate new types of hybrid protocells based on the spontaneous self-assembly of a continuous fatty acid multilamellar membrane at the surface of preformed coacervate microdroplets that comprise molecularly crowded interiors. The approach integrates notions of membrane-mediated compartmentalization, chemical enrichment and internalized structuration, and brings together alternative models of prebiotic compartmentalization based on either phase separated liquid droplets or fatty acid vesicle self-assembly. Our studies indicate that the membrane-enclosed coacervate micro-compartments are semipermeable and exhibit uptake and exclusion properties different to those of the uncoated microdroplets. Thus, it should be possible to exploit the hybrid protocell model to develop transmembrane control of coacervate-sequestered reaction systems such as gene-directed protein synthesis or RNA catalysis by diffusion-limited regulation of the uptake of small molecules from the external continuous aqueous phase.
Small-molecule Uptake in Membrane-free Peptide/ nucleotide Protocells:
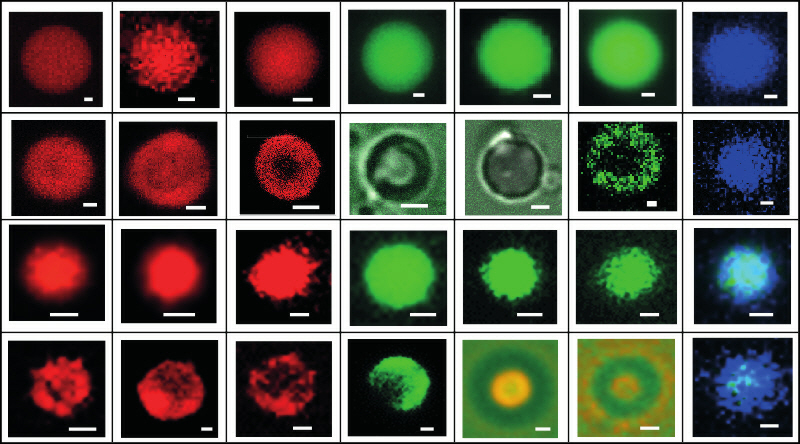
Our investigations show that the anionic fluorescent dye 8-anilionapthalene sulphonate (ANS) can be readily sequestered into the interior of positively charged oligolysine/ATP coacervate micro-droplets to produce a dispersed microphase that exhibits increased morphological stability and complex internal structuration. The uptake of ANS into oligolysine/ATP coacervate microdroplets occurs initially in the outer regions of the droplets, and is associated with a progressive reduction in the zeta potential of the dispersion due to formation of loosely associated ANS/oligolysine/ATP complexes that retain the polar environment and dynamical fluctuations of the native peptide/nucleotide matrix.
Key references:
- Tang T-Y D, Antognozzi M, Vicary J A, Perriman A W and Mann S, Soft Matter, 2013, 9, 7647-7656.
Spontaneous Structuration in Coacervate-based Protocells by Polyoxometalate-mediated Membrane Assembly:
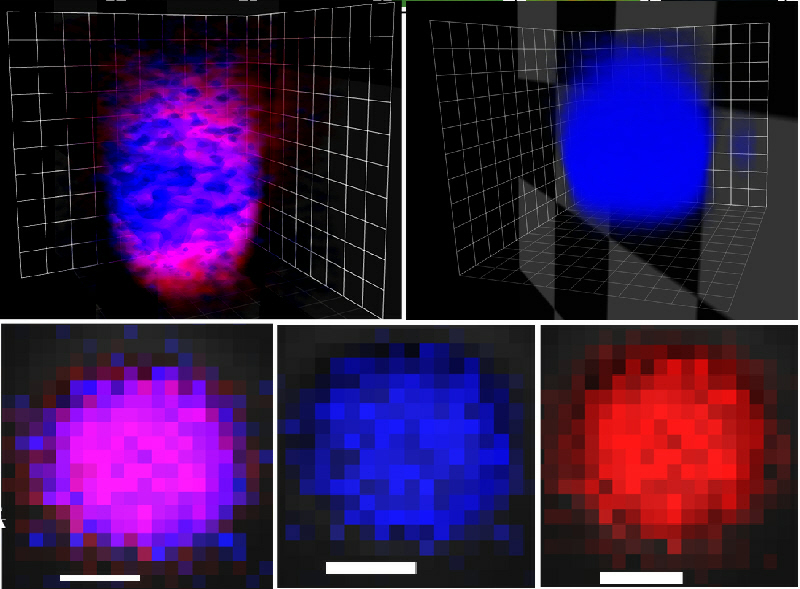
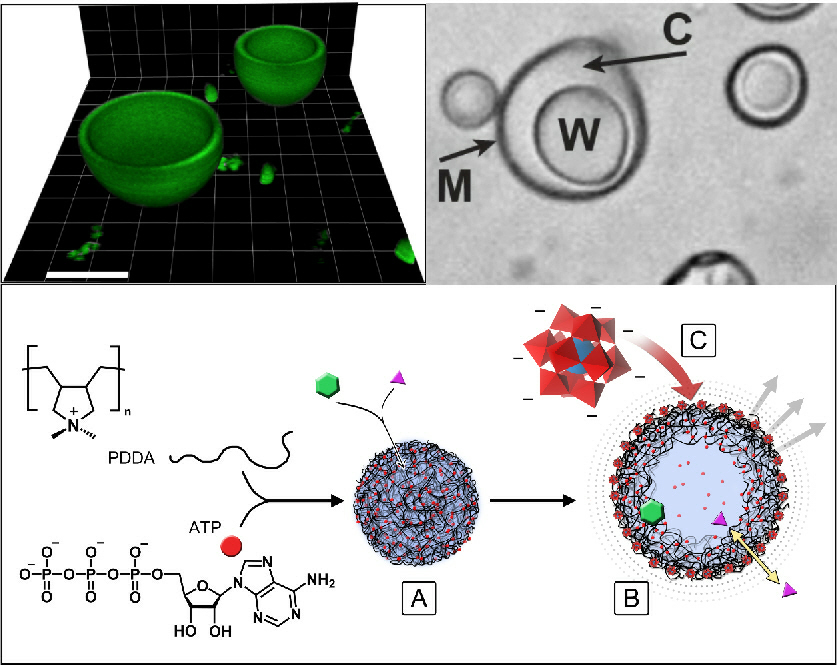
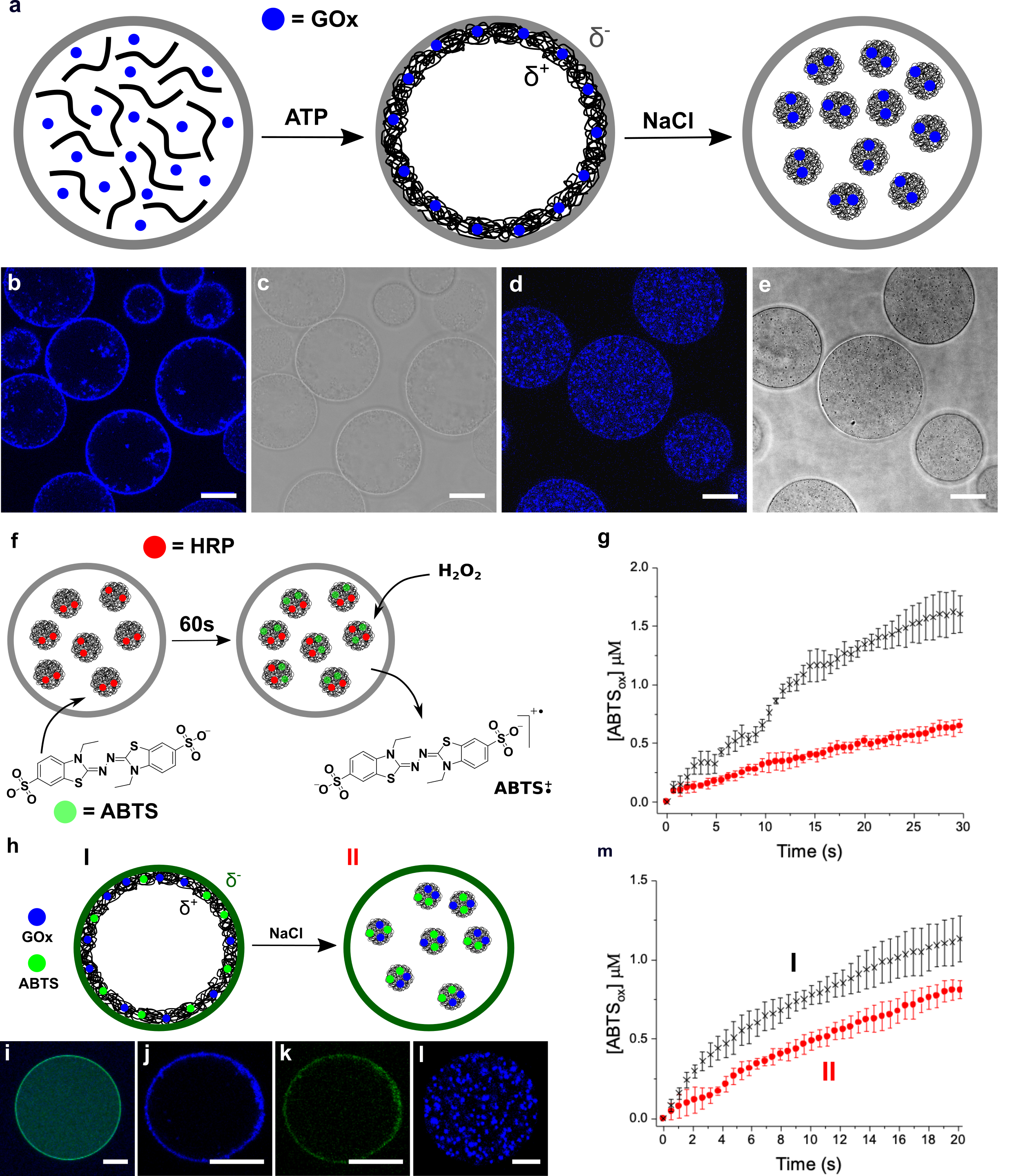
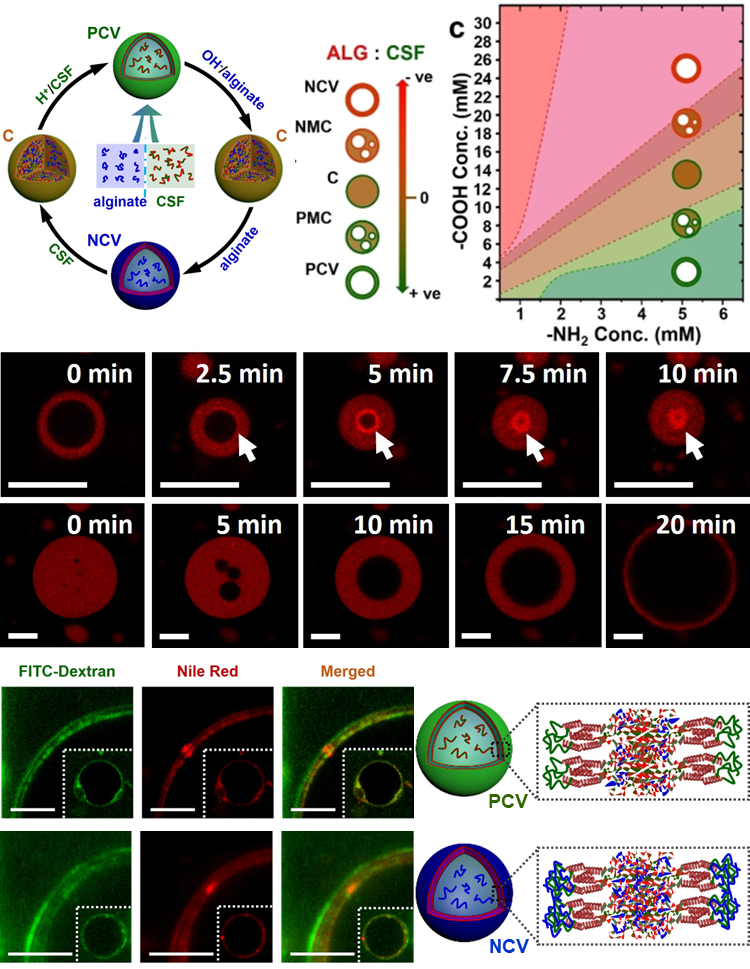
We are also interested in developing hybrid protocells that consist of coacervate micro-droplets enclosed within an inorganic-based membrane. Recently, we demonstrated that molecularly crowded polyelectrolyte/ATP-enriched coacervate droplets can be transformed into membrane-bounded vesicles by using a polyoxometalate-mediated surface-templating procedure. The coacervate to vesicle transition results in spontaneous structuration of the coacervate micro-droplets into novel three-tiered micro-compartments comprising a semi-permeable negatively charged polyoxometalate/polyelectrolyte outer membrane typically 600 nm in thickness, a 2–5 μm-wide sub-membrane coacervate shell capable of sequestering organic dyes, proteins and nanoparticles, and an internal aqueous lumen often tens of micrometres in diameter. The encapsulated proteins are inaccessible to proteases in the external medium, and can be exploited for the spatial localization and coupling of enzyme cascade reactions that are generated within single or between multiple populations of the coacervate vesicles. In particular, by locating different enzymes in different protocell populations, we produce enclosed reaction compartments that can communicate chemically at a distance, provided that the reaction intermediates can diffuse across the boundary membrane.
The integration of molecularly crowded micro-environments into membrane-enclosed protocell models represents a step towards more realistic representations of cellular structure and organization. Herein, the membrane diffusion-mediated nucleation of negatively or positively charged coacervate micro-droplets within the aqueous lumen of individual proteinosomes is used to prepare nested hybrid protocells with spatially organized and chemically coupled enzyme activities. The location and reconfiguration of the entrapped droplets are regulated by tuning the electrostatic interactions between the encapsulated coacervate and surrounding negatively charged proteinosome membrane. As a consequence, alternative modes of a cascade reaction involving membrane- and coacervate-segregated enzymes can be implemented within the coacervate-in-proteinosome protocells.
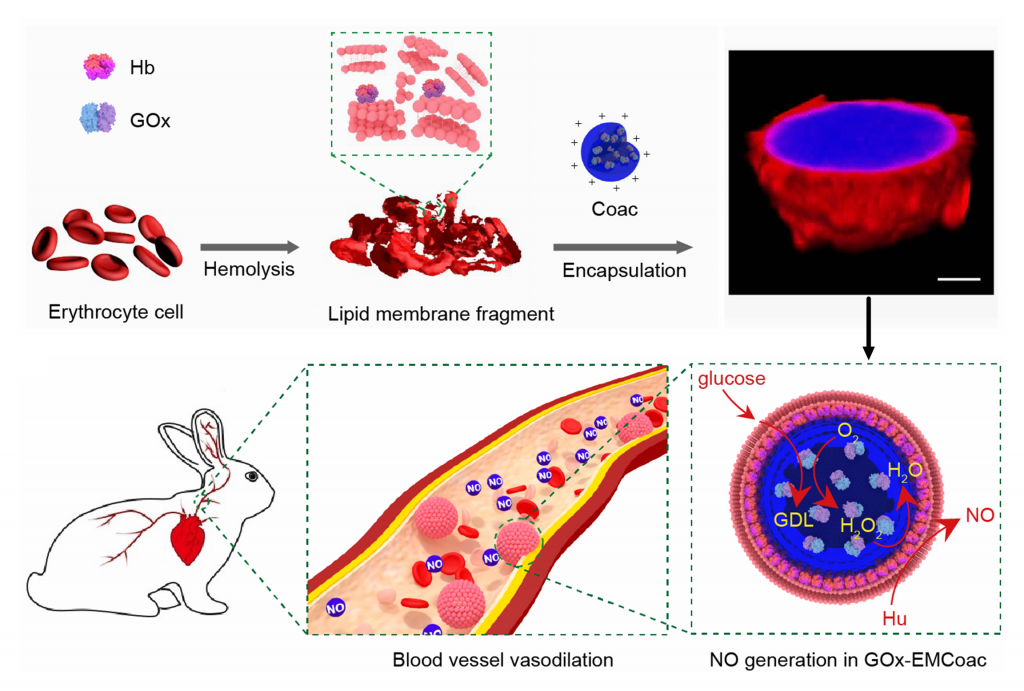
Enzyme-mediated nitric oxide production in vasoactive erythrocyte membrane-enclosed coacervate protocells:
The design and construction of synthetic therapeutic protocells capable of establishing cognate chemical communication channels with living cells is an important challenge for synthetic biology and bio-engineering. Here we develop a step towards protocell-mediated nitric-oxide-induced vasodilation by constructing a new synthetic cell model based on bio-derived coacervate vesicles with high haemocompatibility and increased blood circulation times. The hybrid protocells are prepared by the spontaneous self-assembly of haemoglobin-containing erythrocyte membrane fragments on the surface of preformed DEAE-dextran/dsDNA coacervate micro-droplets containing glucose oxidase. We use the sequestered enzymes to program a spatially coupled glucose oxidase/haemoglobin reaction cascade, which in the presence of glucose and hydroxyurea generates a protocell-mediated flux of nitric oxide that we exploit for in vitro and in vivo blood vessel vasodilation. Taken together, our results provide new opportunities for the development of endogenously organized cell-like entities (biocompatible micro-bots) geared specifically towards active interfacing with individual living cells and cell communities.
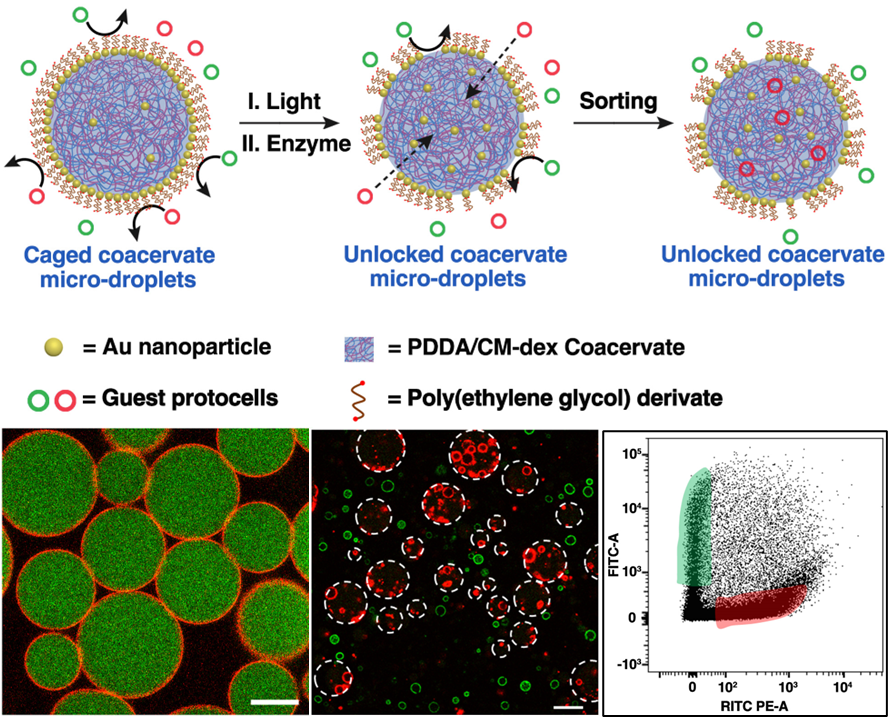
We develop a caged protocell model with a molecularly crowded coacervate interior surrounded by a non-cross-linked gold (Au)/poly(ethylene glycol) (PEG) nanoparticle-jammed stimuli-responsive membrane. The jammed membrane is unlocked by either exogenous light-mediated Au/PEG dissociation at the Au surface or endogenous enzyme-mediated cleavage of a ketal linkage on the PEG backbone. The membrane assembly/disassembly process is used for the controlled and selective uptake of guest protocells into the caged coacervate microdroplets as a path toward an all-water model of triggerable transmembrane uptake in synthetic protocell communities. Active capture of the guest protocells stems from the high sequestration potential of the coacervate interior such that tailoring the surface properties of the guest protocells provides a rudimentary system of protocell sorting. Our results highlight the potential for programming surface-contact interactions between artificial membrane-bounded compartments and could have implications for the development of protocell networks, storage and delivery microsystems, and microreactor technologies.
Key references:
- Ning Gao, Can Xu, Zhuping Yin, Mei Li, Stephen Mann, J. Am. Chem. Soc., 2022, 144, 3855-3862
Key references:
- Zhuping Yin, Liangfei Tian, Avinash J. Patil, Mei Li, Stephen Mann, Angew. Chem. Int. Ed.,, 2022, 61, e2022023
Spontaneous Membranization in Silk-Based Coacervate Protocell Model:
Molecularly crowded coacervate micro-droplets are useful protocell constructs but the absence of a physical membrane limits their application as cytomimetic models. Auxiliary surface-active agents have been harnessed to stabilize the coacervate droplets by irreversible shell formation but endogenous processes of reversible membranization have received minimal attention. Herein, we describe a dynamic alginate/silk coacervate-based protocell model in which membrane-less droplets are reversibly reconfigured and inflated into semipermeable coacervate vesicles by spontaneous self-organization of amphiphilic silk polymers at the droplet surface under non-neutral charge conditions in the absence of auxiliary agents. We show that membranization can be reversibly controlled endogenously by programming the pH within the protocells using an antagonistic enzyme system such that structural reconfigurations in the protocell microstructure are coupled to the trafficking of water-soluble solutes. Our results open new perspectives in the design of hybrid protocell models with dynamical structural properties.
Living Material Assembly of Bacteriogenic Protocells:
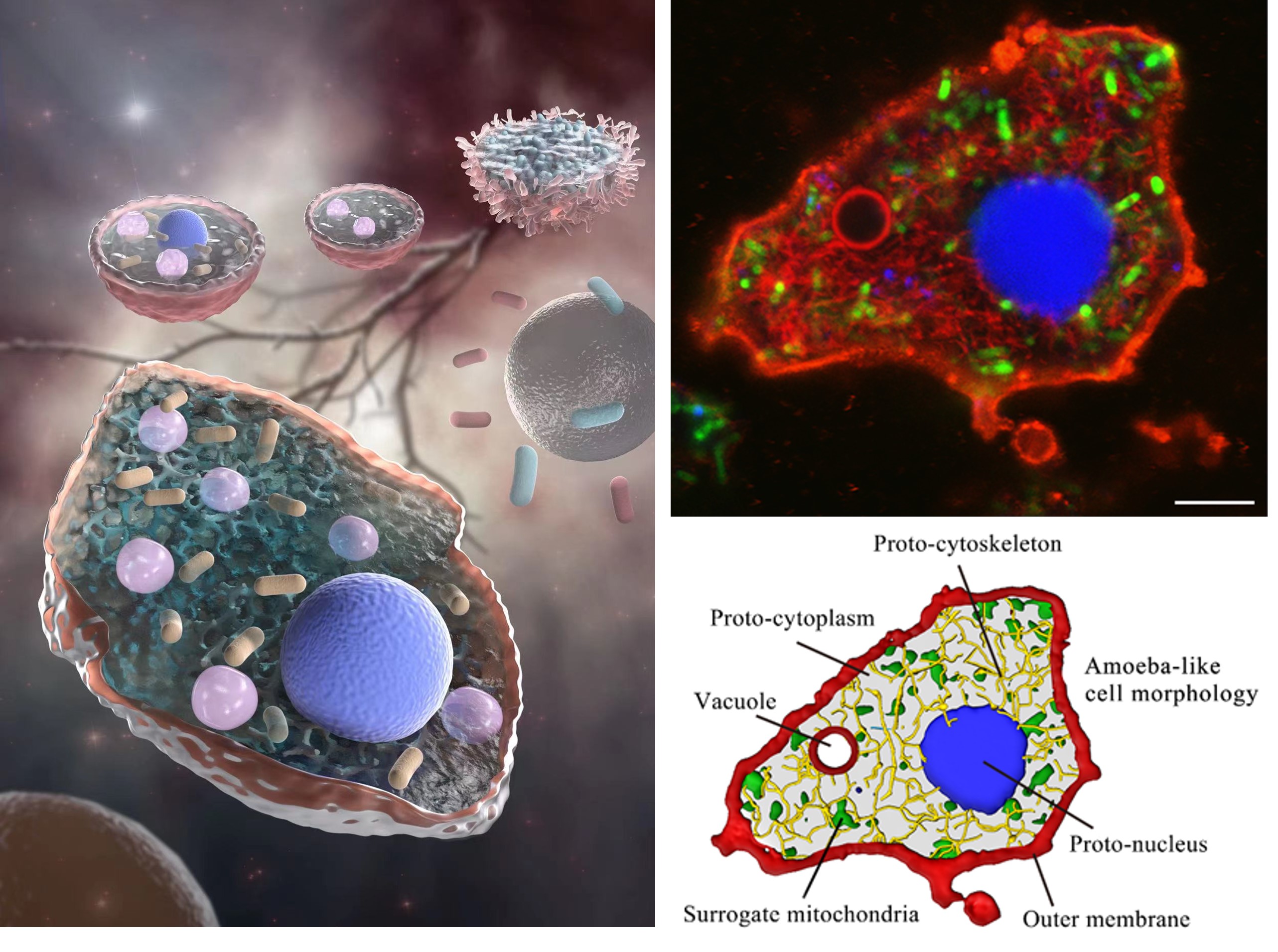
Advancing the spontaneous bottom-up construction of artificial cells with high organizational complexity and diverse functionality remains an unresolved issue at the interface between living and non-living matter1,2,3,4. Here, to address this challenge, we developed a living material assembly process based on the capture and on-site processing of spatially segregated bacterial colonies within individual coacervate microdroplets for the endogenous construction of membrane-bounded, molecularly crowded, and compositionally, structurally and morphologically complex synthetic cells. The bacteriogenic protocells inherit diverse biological components, exhibit multifunctional cytomimetic properties and can be endogenously remodelled to include a spatially partitioned DNA–histone nucleus-like condensate, membranized water vacuoles and a three-dimensional network of F-actin proto-cytoskeletal filaments. The ensemble is biochemically energized by ATP production derived from implanted live Escherichia coli cells to produce a cellular bionic system with amoeba-like external morphology and integrated life-like properties. Our results demonstrate a bacteriogenic strategy for the bottom-up construction of functional protoliving microdevices and provide opportunities for the fabrication of new synthetic cell modules and augmented living/synthetic cell constructs with potential applications in engineered synthetic biology and biotechnology.